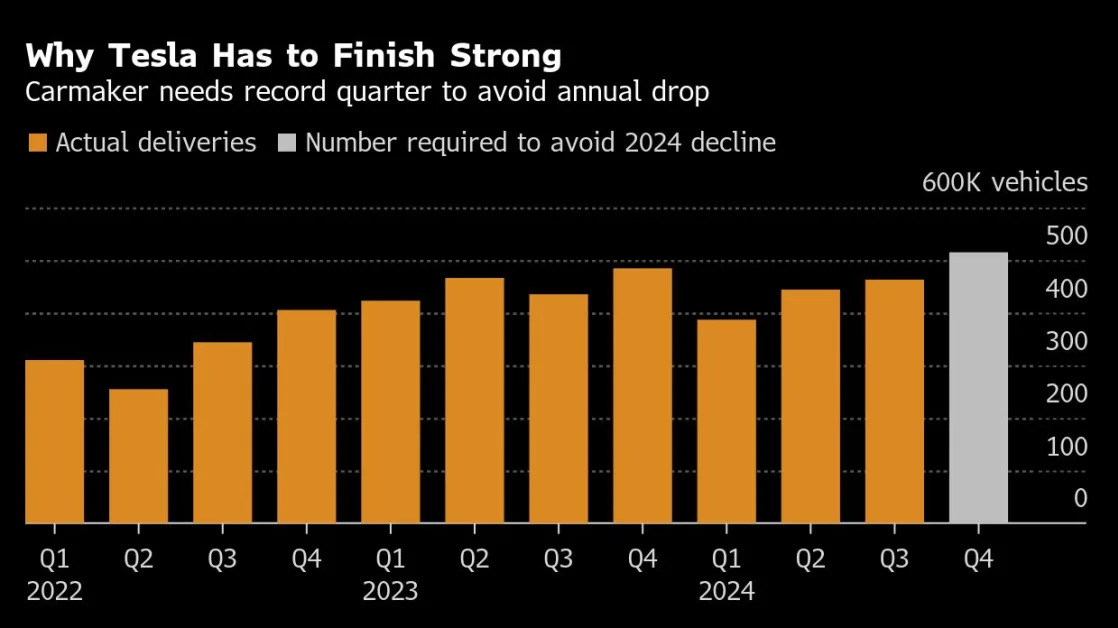
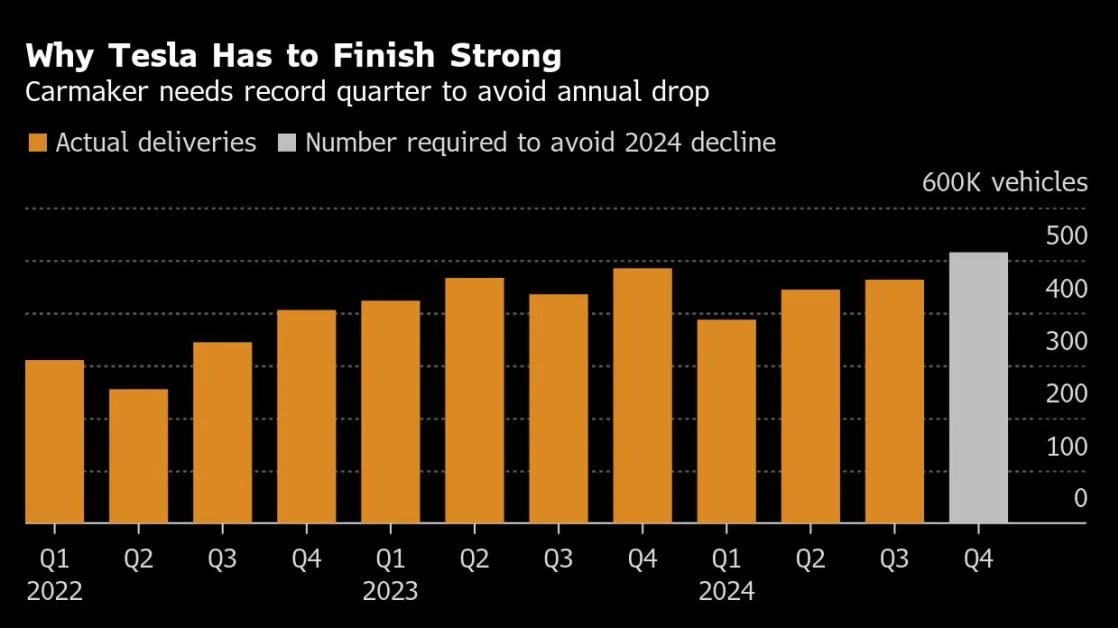
Inogen Expands U.S. Market with FDA Clearance
The latest announcement is out from Inogen ( (INGN) ). Inogen, Inc. announced that it has received FDA 510(k) clearance for its Simeox 200 device, designed to enhance airway clearance and improve bronchial drainage for patients with chronic respiratory diseases. The clearance allows Inogen to expand its market reach in the U.S. and requires the company to make a $13 million milestone payment as part of a previous agreement. This development signifies a step forward in Inogen’s efforts to introdu